INVESTORS
Press Releases
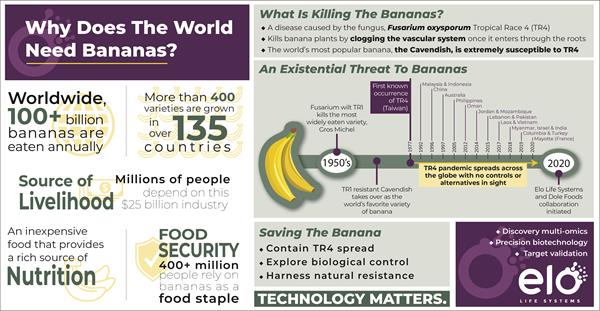
Elo Life Systems Announces Strategic Partnership with Dole Food Company with the Goal of Saving the Cavendish Banana
Fusarium wilt TR4 threatens the $25 billion banana industry without a cure or remedy on the horizon
DURHAM, N.C., Aug. 04, 2020 (GLOBE NEWSWIRE) -- Elo Life Systems, a food and agriculture company with a mission to improve human health and wellness, today announced a strategic partnership with the Dole Food Company, one of the world’s largest producers of high-quality fresh fruit and vegetables, aiming to develop multiple banana varieties, including Cavendish, with resistance to the devastating fungal disease, Fusarium wilt.
Fusarium wilt, caused by the Tropical Race 4 (TR4) strain of a plant pathogenic fungus called Fusarium, is a fast-spreading pandemic threatening the continued cultivation of the world’s most popular fruit in a $25 billion banana industry. The disease was detected in Colombia in August 2019 and is expected to spread throughout Latin America.
“Spread of Fusarium wilt would not only have devastating consequences to the banana industry, but also have a significant economic impact on farmers in the affected regions whose livelihoods depend on exports of the Cavendish banana,” said Elo’s Chief Executive Officer, Fayaz Khazi, Ph.D. “To date, chemical and cultural approaches to control this disease have been unsuccessful. We’re excited to work with Dole, which shares Elo’s vision to improve the security and sustainability of the global food supply, to address this critical need and help develop new varieties that leverage natural resistance within several relatives of the Cavendish banana.”
“Bananas are not only the most popular fruit in the United States, but together with plantains, they are a staple food on which much of the world depends for sustenance,” said Patricio Gutiérrez, Director of Innovation R&D, Dole Tropical Products. “Our investment in this strategic project reflects our aspiration to improve a critically important food crop while helping farmers meet the continuous challenges to produce this food for the planet.”
Under the terms of the partnership agreement, Elo will be primarily responsible for the discovery, evaluation, and development of multiple approaches to achieve resistance to Fusarium wilt, and Dole will be responsible for field evaluation and commercialization of Fusarium TR4 resistant Cavendish varieties. Dole will fully fund the research and development at Elo, in addition to paying royalties on the commercialized plant product.
As part of the collaborative effort, Elo will use its proprietary suite of tools, including cutting-edge knowledge mining platform, gene discovery pipeline, trait validation workflows, and end-to-end expertise in translational agriculture, in combination with its proprietary homing endonuclease-based genome editing platform to develop potential TR4-resistant banana varieties in this important clonally propagated crop.
About Elo Life Systems, Inc.
Elo’s mission is to create novel products that enhance the nutrition and diversity of the global food supply. To address agricultural needs, Elo partners with stakeholders in the food systems value chain to bridge gaps and meet needs across agricultural productivity, nutritional demand, food security, climate-resilience, and human wellness. Elo Life Systems, Inc. is a wholly owned subsidiary of Precision BioSciences, Inc. (NASDAQ: DTIL). To learn more about Elo Life Systems please visit www.elolife.ag.
Forward Looking Statements
This press release contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. All statements contained in this press release that do not relate to matters of historical fact should be considered forward-looking statements, including, without limitation, statements regarding the ability to develop TR4-resistant banana varieties. In some cases, you can identify forward-looking statements by terms such as “anticipate,” “believe,” “could,” “expect,” “should,” “plan,” “intend,” “estimate,” “target,” “mission,” “may,” “will,” “would,” “should,” “could,” “target,” “project,” “predict,” “contemplate,” “potential,” or the negative thereof and similar words and expressions.
Forward-looking statements are based on management’s current expectations, beliefs and assumptions and on information currently available to us. Such statements are subject to a number of known and unknown risks, uncertainties and assumptions, and actual results may differ materially from those expressed or implied in the forward-looking statements due to various important factors, including, but not limited to: our ability to become profitable; our ability to procure sufficient funding and requirements under our current debt instruments; and effects of restrictions thereunder; risks associated with raising additional capital; our operating expenses and our ability to predict what those expenses will be; our limited operating history; the success of our programs and product candidates in which we expend our resources; our dependence on our ARCUS technology; the initiation, cost, timing, progress, achievement of milestones and results of research and development activities, preclinical or greenhouse studies and clinical or field trials; public perception about genome editing technology and its applications; competition in the genome editing, biopharmaceutical, biotechnology and agricultural biotechnology fields; our or our collaborators’ ability to identify, develop and commercialize product candidates; pending and potential liability lawsuits and penalties against us or our collaborators related to our technology and our product candidates; the U.S. and foreign regulatory landscape applicable to our and our collaborators’ development of product candidates; our or our collaborators’ ability to obtain and maintain regulatory approval of our product candidates, and any related restrictions, limitations and/or warnings in the label of an approved product candidate; our or our collaborators’ ability to advance product candidates into, and successfully design, implement and complete, clinical or field trials; potential manufacturing problems associated with the development or commercialization of any of our product candidates; our ability to achieve our anticipated operating efficiencies at our manufacturing facility; delays or difficulties in our and our collaborators’ ability to enroll patients; changes in interim “top-line” and initial data that we announce or publish; if our product candidates do not work as intended or cause undesirable side effects; risks associated with applicable healthcare, data protection, privacy and security regulations and our compliance therewith; the rate and degree of market acceptance of any of our product candidates; the success of our existing collaboration agreements, and our ability to enter into new collaboration arrangements; our current and future relationships with and reliance on third parties including suppliers and manufacturers; our ability to obtain and maintain intellectual property protection for our technology and any of our product candidates; potential litigation relating to infringement or misappropriation of intellectual property rights; our ability to effectively manage the growth of our operations; our ability to attract, retain, and motivate key scientific and management personnel; market and economic conditions; effects of system failures and security breaches, effects of natural and manmade disasters, public health emergencies and other natural catastrophic events effects of the outbreak of COVID-19, or any pandemic, epidemic or outbreak of an infectious disease; insurance expenses and exposure to uninsured liabilities; and other important factors discussed under the caption “Risk Factors” in Precision’ Biosciences, Inc.’s Quarterly Report on Form 10-Q for the quarterly period ended March 31, 2020, as any such factors may be updated from time to time in Precision BioSciences, Inc.’s other filings with the SEC, which are accessible on the SEC’s website at www.sec.gov and the Investors & Media page of Precision BioSciences, Inc.’s website at investor.precisionbiosciences.com.
All forward-looking statements speak only as of the date of this press release and, except as required by applicable law, we do not plan to publicly update or revise any forward-looking statements contained herein, whether as a result of any new information, future events, changed circumstances or otherwise.
Investor and Media Contact:
Maurissa Messier
Senior Director, Corporate Communications
mmessier@elolife.ag
A photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/f6b1c188-25ad-49a7-b3c9-c3d2a1f99f34